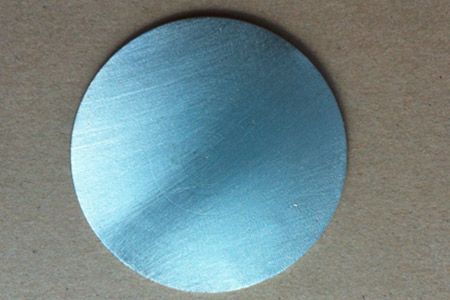
Indium Sputtering Targets (In)


| Material Type | Indium |
| Symbol | In |
| Atomic Weight | 114.818 |
| Atomic Number | 49 |
| Color/Appearance | Silvery Lustrous Gray, Metallic |
| Thermal Conductivity | 82 W/m.K |
| Melting Point (°C) | 157 |
| Coefficient of Thermal Expansion | 32.1 x 10-6/K |
| Theoretical Density (g/cc) | 7.3 |
| Z Ratio | 0.841 |
| Sputter | DC |
| Type of Bond | Elastomer |
| Comments | Wets W and Cu. Use Mo liner. Low Melting Point materials not ideal for sputtering. |
Indium Sputtering Targets
Indium is a chemical element with the symbol In and atomic number 49. It is a post-transition metal that makes up 0.21 parts per million of the Earth's crust. Very soft and malleable, indium has a melting point higher than Sodium and Gallium but lower than Lithium and Tin. Chemically, indium is similar to Gallium and Thallium, and it is mainly intermediate between the two in terms of its properties. Indium is a minor component in Zinc Sulfide ores and is produced as a byproduct of Zinc refinement. It is most notably used in the semiconductor industry, in low-melting-point metal alloys such as solders, in soft-metal high-vacuum seals, and transparent production conductive coatings of indium tin oxide (ITO) on the glass. Indium has no biological role, though its compounds are somewhat toxic when injected into the bloodstream. Most occupational exposure is through ingestion, from which indium compounds are not absorbed well, and inhalation, from which they are moderately absorbed.
Indium Sputtering Targets Information
Indium Sputtering Targets
Purity: 99.99- 99.9999%;
Circular: Diameter <= 14 inch, Thickness >= 1mm;
Block: Length <= 32 inch, Width <= 12 inch, Thickness >= 1mm.
More Information on Indium Sputtering Targets
Applications• Electronics• Semiconductor • Flat panel displays |
Features• Competitive pricing• High purity • Grain refined, Engineered microstructure • Semiconductor grade |
Manufacturing Process• RefiningThree-layer electrolytic process • Melting and casting Electrical resistance furnace - Semi-continuous casting • Grain refinement Thermomechanical treatment • Cleaning and final packaging - Cleaned for use in vacuum Protection from environmental contaminants Protection during shipment |
Options• 99.99% minimum purity• Smaller sizes also available for R&D applications • Sputtering target bonding service |
Related Products of Indium Sputtering Targets
|
Ceramic Sputtering Targets |
||
|
Evaporation Materials |
Crucibles N/A |
Metal Powders N/A |
 Click to download datasheet about Indium Sputtering Targets (In)
Click to download datasheet about Indium Sputtering Targets (In)
 Unable to find the required data sheet? Click here to send an email and get it.
Unable to find the required data sheet? Click here to send an email and get it.
 Click here to get answers to Frequently Asked Questions (FAQ).
Click here to get answers to Frequently Asked Questions (FAQ).
Related Products
FREE QUOTE